The Electrostatic Hypothesis of Hydrogen Water Selectivity in Biological Systems:
Physical and Biological Aspects of Stable Nanobubbles and Water Anomalies
1. Introduction – The Beauty of Simple Observation
For more than twenty-five years, I have been engaged in experimental research on water electrolysis, cold fusion, and the formation of nanobubbles and colloids in water.
During countless observations of electrodes under electrolysis, I noticed a peculiar phenomenon: from the water surface above the electrodes, tiny bubbles were shooting into the air — sometimes up to ten centimeters above the surface.
They were not transparent, but slightly hazy, as if wrapped in a thin film of water — an invisible membrane that gave them both shape and luster.
Although the solution contained no surfactants, the bubbles behaved like microscopic droplets, retaining a smooth surface tension even after leaving the liquid phase.
This naturally raised a simple yet profound question: What holds that delicate water film together once the bubble leaves the surface?
A second observation was equally striking.
While water decomposes into hydrogen and oxygen in a 2:1 ratio, the visual distribution of bubbles was far from proportional. Around the cathode, hydrogen bubbles were not merely twice as numerous — they were orders of magnitude more abundant. They appeared smaller, finer, and formed a milky cloud, whereas at the anode only larger, transparent oxygen bubbles emerged.
video: https://www.youtube.com/shorts/FpfJYYfZQ1Q
Why does the same rule not apply to oxygen?
What causes this asymmetry?
This simple yet puzzling observation led me to a fundamental question: What gives these microscopic bubbles their remarkable stability, their size disparity, and their ability to persist?
It soon became clear that the answer lies not in chemistry alone, but in the microscopic interface between gas and water — the place where electric potential, molecular orientation, and a subtle, still poorly understood electrostatic structure of water come into being.
2. The Gas–Water Interface: The Cradle of the Electric Double Layer
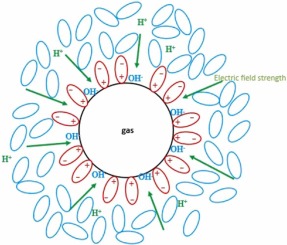
When a gas bubble enters water, an interface immediately forms around its surface — a boundary where the behavior of water molecules changes dramatically.
At first glance, it might appear that the bubble is simply surrounded by ordinary water molecules. In reality, however, the very first layer that envelops it is a thin shell of hydroxide ions (OH⁻).
These negatively charged ions are drawn toward the interface because the presence of a hydrophobic gas bubble disturbs the equilibrium of the water network. This creates a local zone of energetic imbalance — a region where the hydrogen-bond structure of water breaks down.
To minimize its energy, the system compensates by redistributing charge: hydroxide ions rapidly migrate toward the interface, where their presence restores electrostatic balance.
Water molecules near the surface orient themselves so that their positively charged hydrogen atoms point outward toward the gas, while their negatively charged oxygen atoms face inward toward the liquid phase.
This alignment generates an electric field that further stabilizes the layer of hydroxide ions and attracts more water dipoles into an ordered configuration.
The result is a negatively charged bubble surface that behaves like a microscopic electrostatic capacitor. Surrounding it is a broader region of oriented water molecules, arranged according to the direction of the field. Together, these layers form what is known as the Electric Double Layer (EDL) — a delicate yet stable balance between electrostatic repulsion and the polarizing forces of water.
This process occurs within microseconds. Hydroxide ions respond almost instantly to the local imbalance created by the newly formed bubble.
The resulting EDL ensures that the bubbles repel one another rather than merge, which explains their remarkable long-term stability.
In simple terms:
The gas inside the bubble is "wrapped" in a negatively charged shell of hydroxide ions that have moved there to compensate for the energetic imbalance caused by the hydrophobic core.
This ionic shell generates an electric field that holds the bubble together and shields it from coalescing with others.
3. Why Hydrogen Carries the Most Negative Charge
When different gases are dissolved in water, it becomes clear that not all of them form nanobubbles of equal stability.
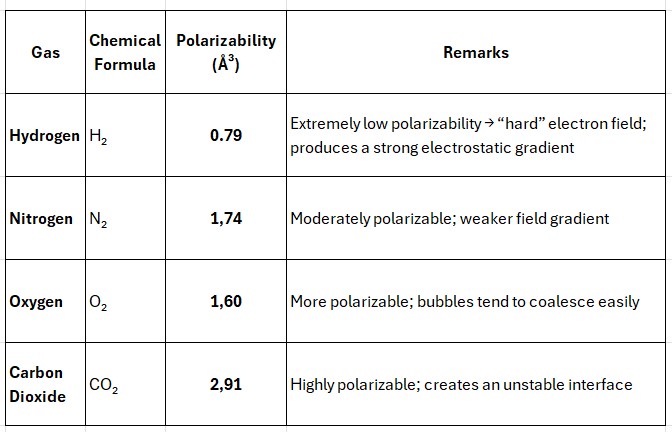
Hydrogen is a remarkable exception. Its bubbles exhibit the highest negative zeta potential (typically −60 to −90 mV), whereas oxygen, nitrogen, or carbon dioxide produce much lower values.
This difference arises from hydrogen's extremely low polarizability — meaning its electron cloud is very compact and difficult to deform by external electric fields.
It is important to emphasize that a hydrogen nanobubble is not composed of a single H₂ molecule but rather of a vast cluster — typically tens to hundreds of thousands of molecules — confined within a gaseous core measuring several tens to hundreds of nanometers in diameter.
Inside such a bubble, a high partial pressure of hydrogen maintains the equilibrium between the gaseous and liquid phases.
Only this collective system of many molecules creates a sufficiently strong hydrophobic contrast with the surrounding water, allowing the formation of a structured Electric Double Layer (EDL) at the interface.
A single hydrogen molecule would not produce a persistent electrostatic effect; such behavior emerges only when a large number of molecules act together, sharing a common electrostatic potential and generating a unified field.
Hydrogen nanobubbles can thus be viewed as collective electrostatic structures.
The compact electron density inside each bubble forces nearby water molecules to orient strongly, attracting hydroxide ions (OH⁻) toward the surface.
This phenomenon gives rise to the negative zeta potential and the exceptional stability of hydrogen nanobubbles.
To restore balance around this charged interface, water molecules and hydroxide ions must undergo significant rearrangement. The result is a highly ordered Electric Double Layer (EDL) with a high density of negative charge, tightly bound and remarkably persistent.
By contrast, gases with higher polarizability — such as oxygen or carbon dioxide — behave more "softly."
Their electrons are easily displaced, which makes the electric field at the gas–water interface weaker and more diffuse.
As a result, hydroxide ions cannot align as closely to the surface, leading to a smaller zeta potential and reduced bubble stability.
In summary:
The proposed electrostatic factor — the observed differences in zeta potential between H₂ and O₂ bubbles suggest that molecular polarizability may also play a role.
Thus, the likely mechanism is that the lower the gas's polarizability, the sharper the gradient of the electric field at its interface with water.
Therefore, hydrogen — the least polarizable element — forms the most stable, most negatively charged, and energetically dense nanobubbles, functioning as miniature reservoirs of electrons capable of maintaining charge and stability for extended periods of time.
4. Scientific Evidence: New Insights into Nanobubble Stability
A recent study by Dukhin & Xu (2024, Colloids and Surfaces A) confirms that the stability of nanobubbles does not primarily depend on the chemical composition of their surface, but rather on dielectrostatic forces and the orientation of water molecules within the Electric Double Layer (EDL).
👉 Read the study on ScienceDirect
The authors describe in detail several key mechanisms that explain the long-term persistence of nanobubbles:
Electrostatic Stabilization:
Nanobubbles persist because of the strong electric field gradient and the adsorption of hydroxide ions (OH⁻) at the gas–water interface, which stabilizes the boundary between the two phases.Counteracting Laplace Pressure:
The surface charge generates an intense normal electric field that counterbalances the internal Laplace pressure—the tendency of small bubbles to shrink spontaneously due to surface tension.Size–Charge Relationship:
Smaller bubbles exhibit higher zeta potentials, which translate into stronger electrostatic stabilization and longer lifetimes.
These findings provide direct scientific support for the electrostatic hypothesis, which posits that the stability of nanobubbles is determined not by surface chemistry, but by the ordered arrangement and orientation of water molecules in the interfacial electric field.
In other words, a nanobubble is not chemically protected but electrostatically organized—and it is precisely this organization that gives it its extraordinary resilience, longevity, and selective response to electric fields of varying polarity and intensity.
5. From Physics to Biology: The Selective Behavior of Hydrogen Nanobubbles
When hydrogen nanobubbles enter the body, they behave as mobile carriers of electrons—microscopic reservoirs of negative charge capable of penetrating even the finest capillaries and cellular environments.
In biological systems, they encounter numerous reactive oxygen (ROS) and reactive nitrogen species (RNS) that arise naturally during metabolism, inflammation, or exposure to toxins.
Mechanism of Electron Transfer at the Bubble Interface
The transfer of electrons during reactions with aggressive radicals—such as the hydroxyl radical (∙OH) or peroxynitrite (ONOO⁻)—takes place at the gas–water interface of the nanobubble.
The source of the electron is not the surface water layer itself, but the molecular hydrogen (H₂) within the gaseous core, which diffuses toward the interface.
Although the reaction between H₂ and the hydroxyl radical also occurs in bulk water, the strong electrostatic field of the EDL may locally lower the activation barrier and thereby increase the likelihood of the reaction.
This catalytic effect remains theoretical, but it corresponds well with the observed high reactivity at the gas–water interface — a hypothesis that some physical chemists are now beginning to consider.
The electrical double layer (EDL) may therefore act as a catalytic environmen - its strong electrostatic field polarizes both the hydrogen molecule and the incoming oxidant, thereby lowering the activation barrier for splitting the H–H bond and enabling a localized redox reaction:
∙OH+H2→H2O+H∙∙OH + H₂ → H₂O + H∙∙OH+H2→H2O+H∙
In this process, one hydrogen atom bonds with the ∙OH radical to form water, while the other remains as a transient hydrogen radical (H∙) that can either react with another oxidant or recombine.
The entire process occurs within the thin, structured EDL, where water molecules are strongly oriented and stabilize the charge transfer.
The molecular hydrogen inside the bubble functions as a reservoir of reducing potential.
As some H₂ at the interface reacts, new molecules diffuse from the bubble's core toward the surface—continuously recharging the interfacial potential.
Thus, a nanobubble can neutralize multiple radicals in succession without losing its stability or integrity.
Summary of the Principle
The electron does not originate from free surface states of water, but from the chemical reaction
H2→2H∙H₂ → 2H∙H2→2H∙ occurring within the EDL field.The EDL itself does not conduct electrons; instead, it creates a polarized environment that facilitates charge transfer between H₂ and the oxidant.
A hydrogen nanobubble functions as a micro-reservoir of H₂, maintaining a sustained reducing potential accessible only to strong oxidants.
As a result, the radical is neutralized and transformed into a less reactive form that no longer harms the cell.
After the reaction, the nanobubble reseals itself—its potential slightly reduced but still stable—and remains active for further interactions.
This mechanism suggests that hydrogen nanobubbles act as reusable microscopic antioxidants, rather than as one-time chemical agents.
Molecules Selectively Spared
The selectivity of H₂ nanobubbles is evident in the fact that they do not react with many biologically important molecules, thereby preserving essential balance and cellular signaling mechanisms.
Hydrogen Peroxide (H₂O₂)
• Reason spared: H₂O₂ is a weaker oxidant than ∙OH, with a redox potential too low for reaction with H₂ in the EDL.
• Biological role: Serves as a crucial signaling mediator regulating metabolism, growth, and immune responses. Eliminating it would disrupt cellular communication.Superoxide Anion (O₂∙⁻)
• Reason spared: Carries a negative charge and is electrostatically repelled by the negative zeta potential of the nanobubble.
• Biological role: Plays a regulatory role and is naturally neutralized by the enzyme superoxide dismutase (SOD); nanobubbles do not interfere with this physiological pathway.Nitric Oxide (NO∙)
• Reason spared: Neutral and weakly oxidizing—incapable of breaking the H–H bond.
• Biological role: A key signaling molecule that regulates blood pressure, immune responses, and neural communication. Preserving NO∙ is vital for homeostasis.Endogenous Antioxidants (e.g., Glutathione, Vitamin C, Vitamin E)
• Reason spared: These molecules have low oxidation potentials and stable bonds.
Hydrogen nanobubbles selectively react only with the most aggressive radicals (∙OH, ONOO⁻), leaving the body's defense systems untouched.
Radicals Excluded by Electrostatic Repulsion
From an electrostatic standpoint, certain radicals are physically excluded from the reactive zone because they are repelled by the negatively charged bubble surface.
Superoxide Anion (O₂∙⁻)
• Charge: Negative (1⁻)
• Mechanism of exclusion: The combination of the superoxide's low redox potential and its negative charge creates a dual barrier — both chemical and electrostatic.
As a result, it cannot even approach the surface of the H₂ nanobubble, which further enhances the system's selectivity.
• Outcome: O₂∙⁻ remains completely unaffected and physiologically intact.Peroxynitrite Anion (ONOO⁻)
• Charge: Negative (1⁻)
• Mechanism of exclusion: Similarly, ONOO⁻ is strongly repelled by the negatively charged EDL.
• Note: Although ONOO⁻ possesses a high oxidation potential, this electrostatic repulsion significantly reduces the reaction kinetics, keeping it at a safe distance—a crucial factor in protecting biological structures.
Selectivity as a Biological Advantage
The selective reactivity of hydrogen nanobubbles is a major advantage:
they specifically target the most harmful oxidants—∙OH and ONOO⁻—which are otherwise difficult for the organism to eliminate, while sparing molecules essential for physiological signaling and balance.
This explains why hydrogen-enriched water acts both intelligently and safely:
it neutralizes where it is beneficial and protects where it is necessary.
Metaphorically speaking, a hydrogen nanobubble behaves like an intelligent membrane—recognizing the aggressor and responding only to signals with sufficient energy.
Energetic Explanation of Selectivity
The selective behavior of hydrogen nanobubbles can also be expressed quantitatively through Gibbs free energy (ΔG), which determines whether a reaction proceeds spontaneously.
The simplified relationship between Gibbs free energy and the difference in redox potentials is:
ΔG°=−nFΔE°ΔG° = −nFΔE°ΔG°=−nFΔE°
A reaction is spontaneous when ΔG° < 0, meaning that the potential difference (ΔE°) between the oxidant and H₂ is large enough to overcome the activation energy of H–H bond cleavage.
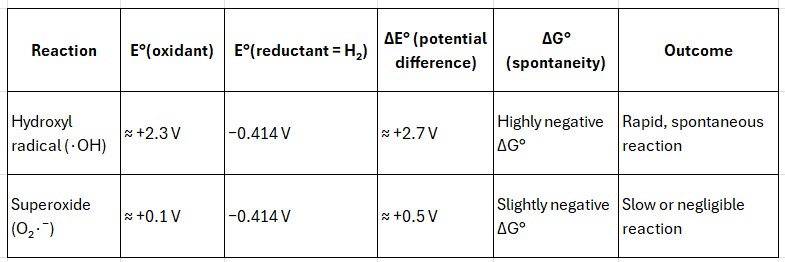
Reaction E°(oxidant) E°(reductant = H₂) ΔE° (potential difference) ΔG° (spontaneity) Outcome
Hydroxyl radical (∙OH) ≈ +2.3 V −0.414 V ≈ +2.7 V Highly negative ΔG° Rapid, spontaneous reaction
Superoxide (O₂∙⁻) ≈ +0.1 V −0.414 V ≈ +0.5 V Slightly negative ΔG° Slow or negligible reaction
Conclusion:
The vast potential gap for strong oxidants such as ∙OH results in a large negative Gibbs energy (ΔG° ≪ 0), ensuring a spontaneous and fast reaction.
In contrast, weak oxidants such as O₂∙⁻ possess too small an energy difference (≈ 0.5 V) for an efficient interaction—and are additionally repelled by the EDL.
This energetic relationship precisely quantifies why hydrogen is selective:
it has sufficient reducing power to neutralize only those radicals that truly need it.
6. Experimental Observations and Quantitative Overview
Zeta potential of H₂ nanobubbles: −60 to −90 mV
Zeta potential of O₂/N₂ bubbles: −20 to −60 mV
Redox pair H₂/H⁺ (theoretical): −0.414 V (at pH 7)
Hydroxyl radical (∙OH): E° ≈ +2.3 V
Peroxynitrite (ONOO⁻): E° ≈ +1.4 V
Superoxide (O₂⁻•): E° ≈ −0.1 to +0.1 V
Nitric oxide (NO•): E° ≈ 0 to +0.8 V
From these values, it becomes clear that hydrogen nanobubbles react spontaneously only with oxidants whose redox potential exceeds roughly +1.0 V relative to the potential of the H₂/H⁺ pair (−0.414 V).
At lower potentials, the energy difference is insufficient to drive spontaneous electron transfer, so weaker radicals—such as NO∙ or O₂∙⁻—remain chemically inactive.
Only strong oxidants can effectively interact with the bubble surface, while weaker radicals "cannot reach the electron."
In addition, the electrostatic barrier of the nanobubble repels these charged species, preventing close contact altogether.
6.1 Selectivity Determined by Redox Potential
Strong oxidants such as the hydroxyl radical (∙OH) and peroxynitrite (ONOO⁻) possess high positive standard potentials (E° > +1 V).
Their oxidative strength is sufficient to break the covalent H–H bond of molecular hydrogen; thus, at the bubble interface, a local redox reaction occurs where H₂ serves as the source of the electron pair.
Through this mechanism, these aggressive radicals are neutralized.
In contrast, weaker radicals such as superoxide (O₂∙⁻) and nitric oxide (NO∙) have much lower redox potentials (close to zero or even negative).
Their oxidative strength is inadequate to react effectively with hydrogen—they simply lack the energy to cleave the H–H bond.
Consequently, they remain chemically inactive toward H₂.
6.2 Selectivity Determined by the Electrostatic Barrier
Beyond redox energetics, electrostatic repulsion plays a decisive role.
Hydrogen nanobubbles carry a highly negative surface potential (−60 to −90 mV).
Negatively charged species—such as superoxide (O₂∙⁻) or the peroxynitrite anion (ONOO⁻)—are therefore electrostatically repelled, preventing them from approaching the reactive interface where they could otherwise interact with H₂.
This dual mechanism establishes both an energetic and a physical barrier:
Weak radicals lack sufficient oxidative power to drive the reaction.
Negatively charged radicals are simultaneously repelled by the bubble's surface charge.
The most prominent radical excluded in this way is the superoxide anion (O₂∙⁻).
The strong negative zeta potential (−60 to −90 mV) of the H₂ nanobubble repels it so effectively that the superoxide cannot reach the interface and remains inactive.
Similarly, the peroxynitrite anion (ONOO⁻), although possessing a high oxidation potential, is also repelled—the electrostatic barrier drastically slows its reaction kinetics, thus protecting biological structures from unwanted reactions.
6.3 Summary of the Selective Mechanism
For weaker, charged radicals, the obstacle is twofold:
both insufficient chemical strength and electrostatic repulsion.
This dual selectivity explains why hydrogen nanobubbles act with such precision and safety—reacting only with the most aggressive oxidants, while leaving weaker and physiologically important species untouched.
The Significance of the H₂/H⁺ Redox Potential
The potential of −0.414 V (at pH 7) defines the energy level from which a hydrogen nanobubble can donate electrons.
The greater the difference between this potential and that of a given oxidant, the more spontaneously electron transfer occurs:
Strong oxidants (∙OH, ONOO⁻) with potentials > +1 V readily overcome this difference and acquire electrons.
Weak oxidants (NO∙, O₂⁻•) with potentials near 0 V lack sufficient driving force and are further repelled by the electrostatic barrier.
This difference explains the selective reductive behavior of hydrogen nanobubbles in biological environments:
they react only where it is both energetically favorable and electrostatically permitted.
6.4 Physical Model of Stability According to Nirmalkar et al. (2018)
The study by Nirmalkar, Pacek & Barigou (Soft Matter, 2018) experimentally confirmed that the stability of nanobubbles is not governed by the chemical presence of surfactants, but by the balance of forces between Laplace pressure and the electrostatic pressure generated by the Electric Double Layer (EDL).
👉 Read the study here
The surface charge of the bubble (σ) creates an electrostatic field that counteracts the internal Laplace pressure:
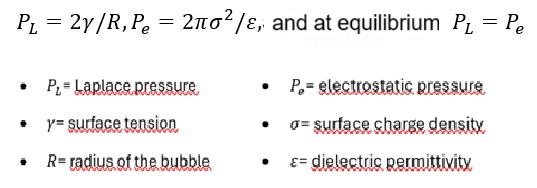
This explains why nanobubbles with diameters between 50–300 nm remain stable for days or even weeks:
their negative surface potential effectively balances the internal gas pressure.
At the same time, this electrostatic field acts as a selective barrier, determining which ions or radicals can approach the interface and participate in reactions.
"The external electrostatic pressure created by the charged nanobubble interface balances the internal Laplace pressure; therefore, no net diffusion of gas occurs at equilibrium and the nanobubbles are stable."
(Nirmalkar et al., Soft Matter, 2018, 14, 9643–9656)
This model provides a solid physical foundation for the electrostatic hypothesis of hydrogen-water selectivity:
the stability and selectivity of nanobubbles are two aspects of the same equilibrium between electrostatic and chemical forces.
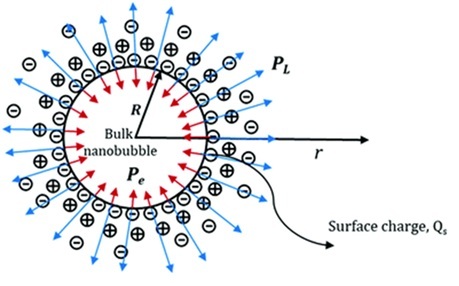
[Figure 6.1]
Balance of forces acting on a nanobubble — Laplace pressure (inward arrows) vs. electrostatic pressure of the EDL (outward arrows).
Adapted from Nirmalkar et al., Soft Matter, 2018, 14, 9643–9656.
6.5 Experimental confirmation of nanobubble stability (Dukhin & Xu, 2024)
Dukhin, S. S., & Xu, Q. (2024). Electrostatic origin of nanobubble stability: Role of hydroxide ions and water dipole orientation.Colloids and Surfaces A: Physicochemical and Engineering Aspects, 708, 133516.
The study provides crucial experimental evidence supporting the electrostatic nature of nanobubble stability.
The authors discovered that even in systems where gases were assumed to be fully dissolved, they actually persist as stable nanobubbles of extremely small size.
Their findings demonstrate that nanobubble stability is not governed by chemical surfactants, but by electrostatic forces acting at the gas–liquid interface.
Adsorbed hydroxide ions (OH⁻) and oriented water dipoles generate an electric field that counterbalances the internal Laplace pressure, allowing the nanobubbles to remain stable for days or even weeks.
This result directly supports the electrostatic hypothesis, confirming that the balance between Laplace pressure and EDL electrostatic pressure is the key to long-term nanobubble stability. https://www.sciencedirect.com/science/article/abs/pii/S092777572401669
6.6 Physicochemical approach to nanobubble solutions
Zhang, X., Zhang, J., & Matsumoto, M. (2010). Physicochemical approach to nanobubble solutions. Chemical Engineering Science, 65(9), 1833-1842. Available online ScienceDirect
Key message:
The authors investigated nanobubble solutions and found that even when gases are assumed to be fully dissolved, nanobubbles of extremely small average radius and elevated internal pressure exist. Their experiments identify critical parameters such as bubble size, supersaturation, and internal pressure, indicating that classical dissolution theory cannot account for the long lifetimes observed. https://www.sciencedirect.com/science/article/abs/pii/S0009250909007052
7. Synergy with Natural Systems: Living Water and Electrostatic Harmony
The same laws observed in laboratory studies of hydrogen nanobubbles also apply—often in remarkably similar form—to natural systems.
Water, whether in plants, springs, or living organisms, is not a passive medium but an electrostatically organized substance, where the orientation of molecules, the presence of dissolved gases, and subtle mineral components all play essential roles.
The same principles that ensure the stability of hydrogen nanobubbles operate in "living water"—that is, water naturally energized through motion, vortices, and contact with air and minerals. Nature itself "charges" and structures water through electrostatic equilibrium.
7.1 Water in Plant Juices and the Electrostatic Stabilization of ORP
When I infused plant juices with hydrogen, I observed an exceptional resistance of their redox potential (ORP) to temporal degradation.
While pure water saturated with hydrogen (at pH 7 and ~1.6 ppm) typically loses more than 100 mV of negative ORP within 24 hours, juices—especially from cucumbers, but also from carrots, beets, oranges, or coconuts—showed a drop of less than 5 mV after the same period.
This represents more than a tenfold increase in ORP stability.
I attribute this phenomenon to the electrostatic and colloidal stabilization of the electric double layer (EDL) surrounding the hydrogen nanobubbles.
Plant juices create a natural electrostatic environment where water, organic molecules, and minerals form a synergistic system capable of maintaining redox balance over extended periods.
Electrostatic Explanation of Stability
Natural amphiphilic compounds—such as amino acids, carboxylic acids, polyphenols, and chlorophyll—accumulate at the gas–liquid interface, acting as biological surfactants.
They reduce hydrogen diffusion from the nanobubbles and stabilize the surface potential by preventing the collapse of the EDL.
Minerals present in the juices (K⁺, Mg²⁺, Ca²⁺) do not disrupt the charge balance; instead, they act as gentle stabilizers of the EDL.
They partially neutralize the surface charge while preserving the dipolar orientation of water molecules—thereby reinforcing the zeta potential.
Polyphenols and chlorophyll function as natural electron buffers—temporarily storing excess electrons and releasing them gradually back into the system, thus maintaining a stable redox equilibrium without sharp fluctuations in ORP.
The dipolar layers of water remain more firmly oriented due to these organic molecules, which dampen electric-field fluctuations.
The result is a more stable and resilient EDL, with minimal loss of electrostatic potential over time.
Experimental Observations
Measured values confirm that:
Pure water loses > 100 mV of negative ORP within 24 hours.
Cucumber juice shows a decline of < 5 mV / 24 h.
→ ORP stability increases by more than a factor of ten.
This difference is not merely chemical but fundamentally electrostatic.
Cucumber juice forms a natural hybrid colloidal system, in which hydrogen nanobubbles are surrounded by bioactive molecules that sustain their charge and polarization.
Resulting Model
Plant juices create an environment that can be described as a "living electrostatic liquid":
Hydrogen provides electrons,
Water serves as their carrier,
Organic and mineral substances act as stabilizers maintaining the overall equilibrium.
This synergy allows hydrogen nanobubbles to persist far longer in plant extracts than in pure water.
Such findings open a promising area for exploring the biological compatibility and long-term stability of nanobubbles in natural environments—such as plant sap or intracellular fluids.
7.2 Energetic Meaning of ORP and the Role of the Electric Double Layer
Through extended discussion and consultation (with both Gemini and ChatGPT), a crucial functional question emerged:
Which parameter matters more—hydrogen concentration (ppm) or redox potential (ORP)?
Within the framework of neutral pH and the electrostatic hypothesis of nanobubbles, the two parameters are closely linked but physically distinct in meaning.
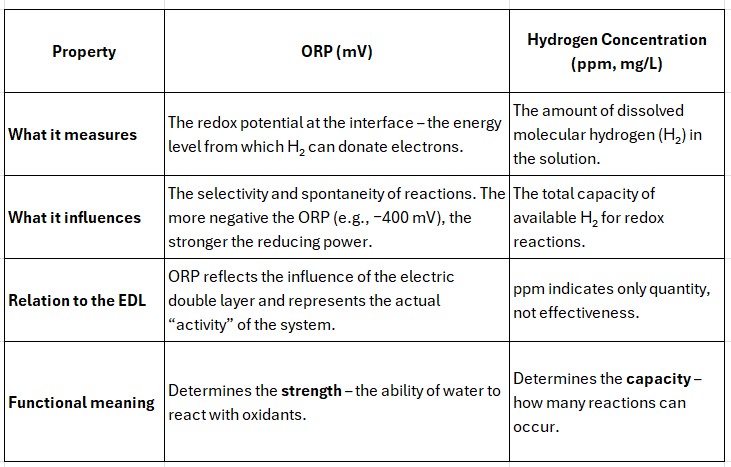
From a Physical Perspective
ORP expresses the energy level of the H₂/H⁺ pair, determining whether a reaction with a given oxidant is spontaneous.
The lower (more negative) the ORP, the greater the potential difference between H₂ and the radical—thus the faster the electron transfer.
In simple terms:
ppm of hydrogen represents the amount of "fuel",
ORP represents its octane rating.
High ppm without a negative ORP does not guarantee antioxidant activity: hydrogen molecules may be physically present but not electrostatically activated.
Conversely, a sufficiently negative ORP indicates that the system is energetically ready for selective reduction.
Influence of Electrolysis and Vortex Flow on the EDL Charge
The charge density of the Electric Double Layer (EDL) can be increased in two main ways:
⚡ During Electrolysis
An increase in pH raises the concentration of OH⁻ ions, which adsorb at the gas–water interface and enhance the negative zeta potential.
The choice of electrolyte (e.g., phosphates or citrates) can strengthen the adsorption of negative ions and thereby increase the charge density of the EDL.
🌪️ During Vortex (Mechanical Generation of Nanobubbles)
Shear stress, cavitation, and bubble collisions lead to triboelectrification – the mechanical separation of charges at the gas–water interface, creating a natural electric field.
At this interface, electrons can be released from water molecules or from the walls of the vessel (if conductive).
The resulting local electric fields inject electrons into the vicinity of the bubbles, where they can become trapped at the bubble surface, further enhancing the negative potential of the EDL.
In other words, the vortex introduces a "living current" of electrons that complements and amplifies the chemically formed EDL.
🔋 Result – Hybrid EDL
Thus, two types of negative charge coexist:
-
Chemically adsorbed (OH⁻, Cl⁻, citrates, etc.)
-
Physically introduced (free electrons from triboelectrification)
These components can synergistically reinforce each other:
The OH⁻ layer provides a stable matrix (electrostatic anchoring),
while the electrons contribute redox activity (increasing the energetic potential → lowering ORP).
💡 Practical Implications
A vortex can increase the negative ORP even without electrolysis,
thanks to the injection of electrons into the EDL.
If the water contains polyphenols, amino acids, or other organic stabilizers,
these substances help capture the electrons and extend their lifetime.
The result is a much more active and stable nanobubble structure,
which behaves like a microscopic redox battery.
In both cases, the negative zeta potential increases,
thereby enhancing the electrostatic stability and selectivity of the nanobubbles.
Conclusion
In systems at neutral pH, ORP has greater functional significance than the hydrogen concentration (ppm), because it reflects the actual energetic readiness of the system to transfer electrons.
ppm represents the quantity,
but ORP represents the capability—and it is this capability that defines selectivity and thus biological efficacy.
From the standpoint of the electrostatic hypothesis, the ideal state is water with a highly negative ORP (−400 mV or lower) and a sustainably structured EDL that preserves long-term nanobubble activity without loss of stability.
7.3 Schauberger's Water and the Role of Colloidal Minerals
The Austrian natural scientist Viktor Schauberger observed that water in natural vortices—springs, streams, and waterfalls—appears to gain "vitality" and self-organization.
From today's scientific perspective, his descriptions can be interpreted as electrostatic phenomena arising from the combination of vortex motion, dissolved atmospheric gases (O₂, N₂, CO₂), and the presence of colloidal minerals.
During vortex motion, pressure and electric field gradients develop, promoting the formation of microscopic gas nuclei—nanobubbles—while simultaneously supporting the adsorption of mineral ions onto their surfaces.
These particles act as natural electrostatic capacitors, capturing charge, polarizing surrounding water molecules, and stabilizing the delicate interface between the liquid, gas, and mineral phases.
Colloidal minerals do not behave merely as passive components of the electric double layer (EDL).
They can function as micro-electrostatic nodes in their own right—similar to nanobubbles.
Each colloid carries its own surface charge and forms a structured hydration shell around itself.
In combination with dissolved gases (O₂, N₂, CO₂), this gives rise to a complex microsystem network that stores electrical energy and supports the self-organization of water.
Schauberger's "living water" thus represents a natural electrostatic state, in which gas bubbles and mineral colloids act together as elementary units of organization—micro-electrostatic building blocks of life.
A review of scientific literature (at the bottom) from 2023–2025 supports the mechanico-electrostatic model of nanobubbles, in which triboelectrification and the electric double layer (EDL) jointly maintain stability, a negative zeta potential, and redox activity of the system, measurable through ORP.
7.4 The Common Principle of Nature
Whether in plant sap, spring water, or the intracellular environment, the same principle repeats across all natural systems:
the stability and vitality of structure arise from the interaction between negative surface potential, the orientation of water molecules, and the presence of trace ions and gases.
In other words, nature itself employs the principle of electrostatic organization—creating self-maintaining nanostructures through the harmony of charge, motion, and matter.
What we observe in hydrogen nanobubbles is therefore the modern physical expression of an ancient natural law:
life emerges wherever movement, electric fields, and equilibrium coexist.
8. Summary of the Electrostatic Hypothesis
A hydrogen nanobubble is more than just a physical curiosity — it represents a model example of electrostatic organization of matter, a principle observable throughout nature.
Its behavior demonstrates that stability, selectivity, and longevity are not random traits, but direct consequences of an ordered electric field and the orientation of water molecules within it.
In this context, the core principles of the electrostatic hypothesis can be summarized as follows:
Hydrogen nanobubbles are electrostatically stabilized micro-reservoirs of electrons, their surfaces carrying a negative potential (−60 to −90 mV).
The selectivity of their behavior arises from differences in redox potentials — they react only with oxidants whose oxidative strength exceeds +1.0 V relative to the H₂/H⁺ couple (−0.414 V).
Their stability results from the orientation of water molecules within the electric double layer (EDL) and from the repulsive forces between negatively charged interfaces.
Colloidal minerals and dissolved atmospheric gases can form similar micro-electrostatic systems, which interact with nanobubbles and extend their lifetime.
The biological effect lies in their ability to donate electrons selectively — only where it is energetically and biologically appropriate.
Thus, hydrogen nanobubbles can be viewed as intelligent electrostatic antioxidants, acting in harmony with natural processes.
They are not chemical reagents in the conventional sense, but physical regulators of equilibrium — a subtle field that moderates extremes without disturbing the natural dynamics of life.
In essence, a hydrogen nanobubble behaves as a microscopic electrostatic capacitor, protecting living systems from oxidative stress while maintaining redox balance — precisely as nature itself does.
9. Epilogue: Water as the Electrostatic Field of Life
Water — the most common substance on Earth — reveals itself in a new light as an active carrier of energy and equilibrium.
It is not merely a solvent, as classical chemistry once believed, but a dynamic electrostatic medium that organizes its surroundings through dipoles, charges, and stabilizing interfacial structures (EDL).
Hydrogen nanobubbles, colloidal minerals, and gases dissolved in vortexed water — all these systems represent different expressions of the same underlying principle:
the natural self-organization of matter through electric charge.
Each maintains a delicate balance between attraction and repulsion, between chaos and order.
And it is precisely within this balance that life's stability emerges — the ability to store energy and release it where it is needed most.
From this perspective, water is the oldest electrostatic field of life — the universal medium linking physics, chemistry, and biology into a single harmonious system.
Ladislav Peš
Scientific Context and Limitations of the Electrostatic Hypothesis
The electrostatic hypothesis of hydrogen water selectivity represents a theoretical framework that connects the physical and biological aspects of nanobubbles — their stability and selective behavior.
It builds upon established concepts such as the electrical double layer (EDL), zeta potential, Laplace pressure, and the dielectric polarization of water, while extending them through new correlations between interfacial electrostatic structure and biological reactivity.
However, it is important to emphasize that some of the presented conclusions go beyond the current scientific consensus.
Certain relationships — such as the influence of hydrogen's polarizability on zeta potential magnitude or the catalytic role of the EDL in redox reactions — are proposed as hypotheses that require further experimental and theoretical validation.
These hypotheses are formulated with the intention of stimulating further research into electrostatic phenomena at the gas–water interface and their potential biological significance.
Therefore, the proposed model should be understood as an open scientific proposition rather than a closed system of facts.
Its main contribution lies in offering a new perspective that bridges classical physical chemistry with observed phenomena in hydrogen systems — potentially inspiring future experimental studies.
Supporting Scientific Literature (2023–2025)
I. Mechanical Charge Generation (Triboelectrification / Physical Component of the EDL)
Recent studies confirm that dynamic fluid motion at the gas–liquid interface generates and anchors electrical charges, validating the concept that "a vortex adds a living current of electrons."
Topic: Hydrodynamic Cavitation (Vortex) and Zeta Potential
These studies examine how hydrodynamic cavitation parameters influence the surface charge (ζ-potential) and stability of nanobubbles, showing that mechanical energy directly affects the strength of the electric double layer (EDL).
-
Zhang, J., & Li, F. (2020). An experimental study on size distribution and zeta potential of bulk cavitation nanobubbles. Applied Mineral Processing Technology. Retrieved from https://www.researchgate.net/publication/339070966
Topic: Triboelectric Nanogenerators (TENG) at Liquid Interfaces
These works explore the conversion of mechanical energy (e.g., water or bubble motion) into electricity via triboelectrification at the gas–liquid interface, confirming that bubbles and vortex motion can mechanically separate electrical charges.
-
Chen, Y., et al. (2024). Tungsten–liquid triboelectric nanogenerator for water-flowing energy harvesting with low resistance and high DC density. Energy, 309, 133104. https://doi.org/10.1016/j.energy.2024.133104
-
Zhao, X., et al. (2025). Triboelectric nanogenerator based on a moving bubble in liquid for mechanical energy harvesting and water level monitoring. ResearchGate. Retrieved from https://www.researchgate.net/publication/358224740
II. Evidence for Redox Activity and Stability (ORP / Energetic Potential)
These papers demonstrate that nanobubbles possess unusual chemical activity and long-term stability attributed to their strong electric double layer (EDL).
Topic: Stability and Mechanisms of Nanobubbles
A comprehensive review highlighting that the persistence of nanobubbles cannot be explained by classical thermodynamics and that surface charge (EDL) is a key stabilizing mechanism.
-
Wang, L., et al. (2024). The existence and stability mechanism of bulk nanobubbles: A review. Nanomaterials, 15(4), 314. https://www.mdpi.com/2079-4991/15/4/314
Topic: ORP as an Indicator of Nanobubble Reactivity
Although focused on ozonation, this work demonstrates how ORP serves as a real-time indicator of micro/nanobubble reactivity, supporting the "redox battery" concept.
-
Kim, H., et al. (2024). Optimizing micro/nanobubble ozonation: Unveiling key transition points in ozone oxidation through real-time ORP monitoring. ACS ES&T Water. https://pubs.acs.org/doi/10.1021/acsestwater.4c00128
Summary:
Together, these studies support the theoretical framework that:
-
Mechanical motion generates charge through triboelectrification.
-
This charge stabilizes nanobubbles via a hybrid EDL structure.
-
The resulting stability and charge distribution alter electrochemical behavior, measurable through ORP shifts.
